Aluminum Anodized Motorbike Clutch
Aluminum Anodized Motorbike Clutch
Image Description: Several aluminum anodized motorbike clutch parts are neatly arranged on a surface.
Are you frustrated with aluminum products that scratch, corrode, or lose their shine over time? While standard aluminum looks beautiful at first, it does not withstand extreme hot or cold conditions. However, without additional protection, the coating can easily wear off, rust, or fade.
Just think of your aluminum CNC machining parts discoloring, corroding, or even getting weak together. Whether it is our house, car, or industrial equipment, these problems can cause time wastage, money, and a need for repair. So, the need for a superior solution is evident.
Aluminum anodizing appears to be a perfect solution to such challenges. Since it forms a layer of oxide to protect the surface hardness and security against erosion. Furthermore, it improves the appearance of a material. Anodized aluminum is also tough, durable, and has a professional-looking appearance. Therefore, it can stand in difficult conditions and does not tarnish.
What is Aluminum Anodizing?
 Aluminum Anodized Parts In Bulk
Aluminum Anodized Parts In Bulk
Image Description: A collection of aluminum anodized parts in bulk, showing shiny and colorful metal surfaces.
Aluminum anodizing is an electrochemical process that improves the natural oxide layer on aluminum surfaces. In this process, the electric current flows through an acid electrolyte bath while the aluminum is submerged. So, the denser aluminum oxide layer converts the layer to a strong and rust-resistant one.
The anodizing process is different from the natural oxidation process. It usually results in forming a soft, thin surface oxide layer. Surface anodizing is resistant to wear or corrosion. Additionally, manufacturers can dye the anodized layer to enhance the product’s appearance and functionality. The anodized coating is also nonconductive, making it ideal for electronic components.
How Aluminum Anodizing Works?
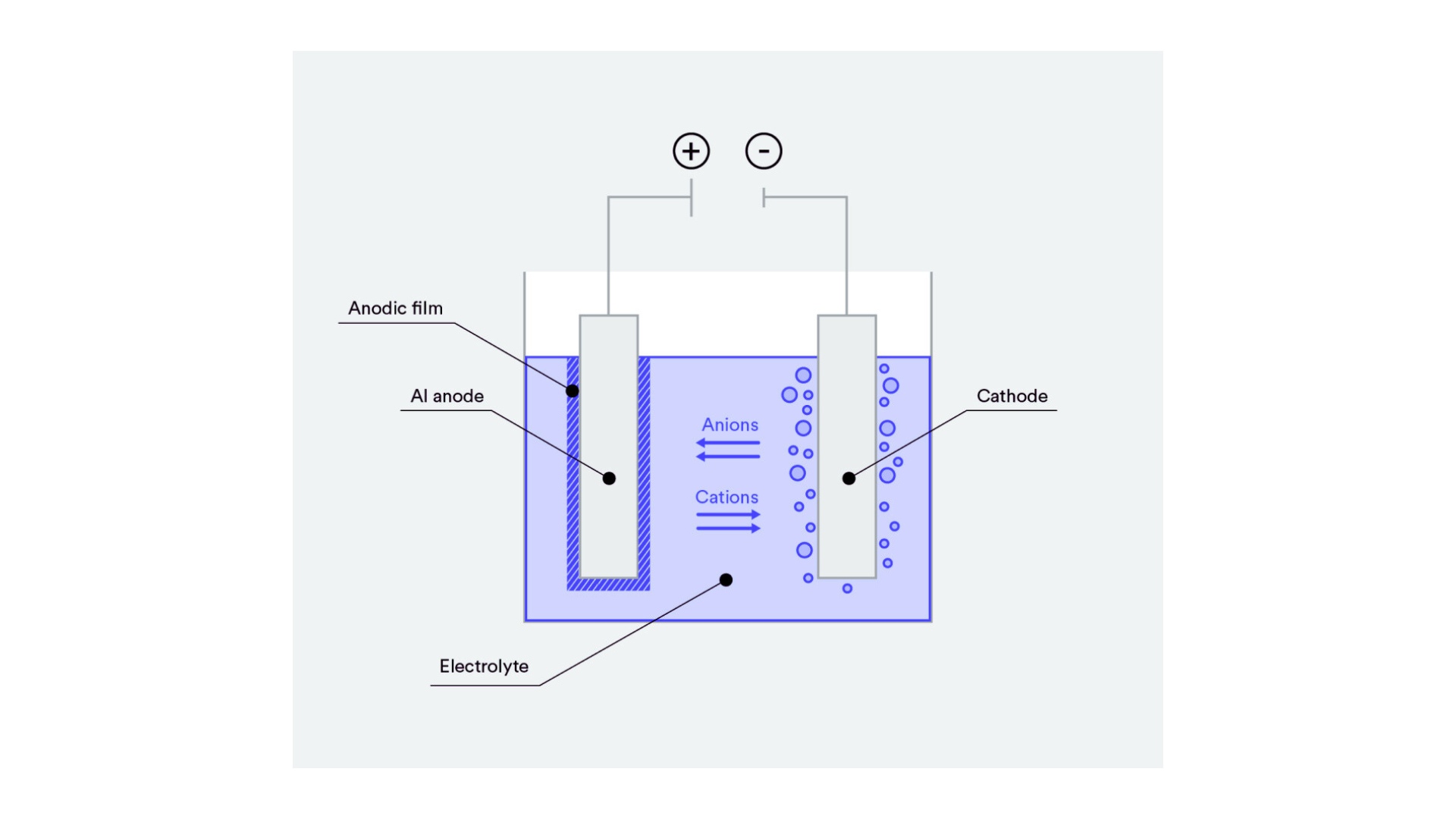 Aluminum Anodizing Process
Aluminum Anodizing Process
Image Description: An illustration showing the aluminum anodizing process with cleaning, anodizing, coloring, and sealing steps.
The aluminum anodizing process comprises various steps for protecting the exterior surface. Let’s break down each step.
1. Cleaning the Aluminum Surface
The first process in anodizing involves degreasing the surface of the aluminum. Aluminum can become dirty, greasy, oiled, or oxidized in the course of its usage. To ensure the anodized layer adheres correctly, workers wash and rinse the metal thoroughly. The most common cleaner to be used at this stage comprises a minor alkaline or acid solution. Afterward, the surface is rinsed to eliminate all remaining traces.
2. Etching
When aluminum is cleaned, it is then etched to eliminate the natural oxide layer to prepare the aluminum for anodizing. It’s done using a mild acid solution, so a combination of sulfuric acid and water is usually preferred. Etching also serves the purpose of producing a uniform surface to facilitate the oxide layer formation on the surface. The intended step could also involve an operation called de-smutting. In de-smutting any remaining contaminants that can cause defects in the anodizing process, they are eliminated.
3. Oxide Layer Formation
Next, workers place the aluminum in an electrolyte bath of sulfuric acid and water. In the course of this phase, the solution is subjected to the passing of an electric current. The aluminum itself serves as the anode, and a cathode is immersed in the solution. As the current flows, oxygen ions are produced at the aluminum surface, and the aluminum starts corroding and a thick, porous oxide layer is formed. The thickness of the oxide layer varies with the process time, current density, and temperature of the electrolyte bath.
4. Optional – Dyeing the Anodized Aluminum
As a result of the structure of the oxide layer, it’s possible to immerse it in various dye solutions. The dyes penetrate the pores of the oxide layer rather than sitting on the surface. The color is infused into the aluminum, making it extremely resistant to fading or chipping off at some point in time. Some of the most frequently used dyes are black, gold, bronze, blue, and red, as per requirement. Once the dye is washed, the aluminum is washed and readied for sealing.
5. Sealing the Oxide Layer
Oxide layer sealing is the last phase of the aluminum anodizing process. Sealing acts to fix the dye in place (if used) and close the pores of the anodized layer, thereby increasing the corrosion protection. It involves the aluminum in boiling water or soaking it in a nickel acetate bath. Besides the improved material lifetime, sealing of the material also increases the hardness and renders the surface less susceptible to scratches. In addition, sealing helps keep out contaminants from penetrating the pores, consequently maintaining a smooth coat and easy-to-clean surface.
6. Final Check and Quality Assurance
Finally, quality control checks the anodized layer on the aluminum to ensure its quality. Manufacturers fix any flaws and ensure the finished product is well-protected for distribution.
Types of Aluminum Anodizing Processes: Type I, Type II, and Type III Explained
The aluminum anodizing process can be classified into three major types, namely the Type I, Type II, and Type III anodizing processes. Here’s a breakdown of each type;
Type 1: Chromic Acid Anodizing (Organic Acid Anodizing)
 Type (I) Anodized Products
Type (I) Anodized Products
Image Description: Type I anodized products with a smooth, matte finish, showing light-colored aluminum surfaces.
Type 1 anodizing employs chromic acid as the electrolyte to form a thin, hard, and corrosion-protected layer of oxide on the aluminum. It is used when protecting the aluminum base is the main concern. The oxide layer is thinner than other types of anodizing, which makes it more appropriate for industries such as aerospace and military.
- Process: Chromic acid anodizing employs a weak electrolyte bath. The process yields a thinner, more uniform oxide layer, which provides outstanding corrosion protection with negligible compromise of the aluminum’s mechanical characteristics.
- Applications: Manufacturers typically use Type I anodizing for applications where weight is crucial and components require high fatigue properties.
Key Characteristics:
- Coating Thickness: Conventional values range from 0.0001” to 0.001” (0.025 mm to 0.0025 mm).
- Finish: Thin, translucent oxide coating.
- Corrosion Resistance: High, but smaller in thickness than other types of earphones.
Type 2: Sulfuric Acid Anodizing
 Type (II) Anodized Product
Type (II) Anodized Product
Image Description: Type II anodized product with a bright, colored surface and smooth aluminum finish.
Sulfuric acid anodizing is also called Type II anodizing. The process immerses the aluminum in an electrolyte bath containing sulfuric acid, producing a denser oxide layer than Type I. The formed oxide layer is thicker and has better protection from corrosion and wear.
- Process: Sulfuric anodizing yields a medium-thickness oxide layer. Moreover, the process is relatively faster than other anodizing processes, hence making it economical to save expense and time.
- Applications: For aluminum window frames and decorative and structural parts, type II can be optimal.
Key Characteristics:
- Coating Thickness: Ranges from 0.0002” to 0.001” (0.005 mm to 0.025 mm) for a precise surface.
- Finish: Matted or satin(a glossy finish or dyed to a specific color)
Type 3: Hard Coat Anodizing (Heavy Duty Anodizing)
 Hard Coat Anodizing (Type III)
Hard Coat Anodizing (Type III)
Image Description: A hard-coated anodized (Type III) aluminum part with a dark, thick, and durable surface finish.
Type III is considerably the most protective and toughest anodizing in the market. It employs concentrated sulfuric acid as an electrolyte at a lower temperature to produce a much heavier and more compact oxide layer than the two former methods. The thickness achieved is usually greater than that of conventional coatings and provides resistance to wear, corrosion, and abrasion.
- Process: Hard coat anodizing creates a thick, durable oxide layer that resists damage from other processes. The process develops an exceptionally hard and strong surface ideal for applications that require sturdy components exposed to mechanical and chemical stresses.
- Applications: OEM Manufacturers use Type III anodizing on parts for aircraft, military equipment, industrial machinery, and high-performance automotive equipment
Key Characteristics:
- Coating Thickness: In general, 0.001 in (0.025 mm) to 0.003 in (0.075 mm) thick, as desired.
- Finish: Usually non-shiny, and dark grey to black.
- Corrosion Resistance: Excellent wear and abrasion resistance.
- Abrasion Resistance: Ideal for components that experience a lot of wear and tear or rubbing.
Comparison of Type 1, Type 2, and Type 3 Anodizing
| Feature | Type 1 (Chromic Acid) | Type 2 (Sulfuric Acid) | Type 3 (Hard Coat) |
| Coating Thickness | 0.0001″ – 0.001″ (0.0025 mm – 0.025 mm) | 0.0002″ – 0.001″ (0.005 mm – 0.025 mm) | 0.001″ – 0.003″ (0.025 mm – 0.075 mm) |
| Finish | Transparent, subtle | Matte or satin can be polished | Dull or matte can be polished to glossy finish |
| Corrosion Resistance | High | Excellent | Exceptional |
| Wear Resistance | Moderate | Good | Superior |
| Common Applications | Aerospace, military, paint prep | Automotive, architectural, and consumer products | Aerospace, military, industrial machinery |
Factors to Look at When Planning for Aluminum Anodizing
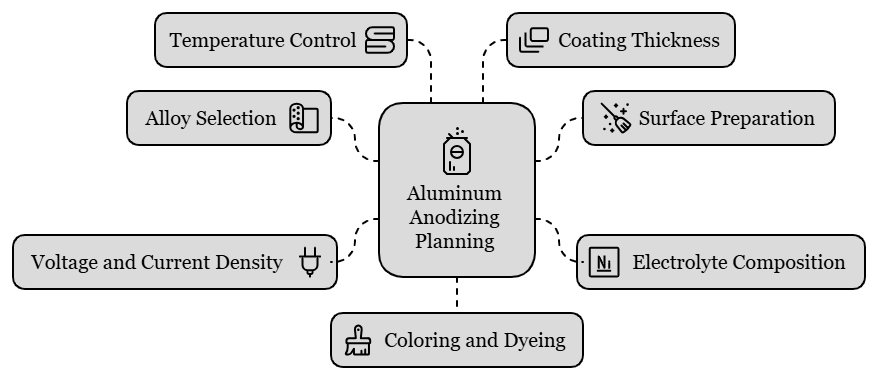 Factors Affecting the Aluminum Anodizing Process
Factors Affecting the Aluminum Anodizing Process
Image Description: An illustration showing different factors that affect the aluminum anodizing process, such as temperature, voltage, and time.
Below are the primary technical elements to consider:
1. Alloy Selection
The oxide layer of aluminum depends on the different aluminum alloy compositions; therefore, the thickness, uniformity, and oxide layer hardness are not the same across all alloys. Manufacturers frequently use 6061 and 5052 alloys for anodizing because these alloys form a dense oxide layer during the treatment. On the other hand, alloys such as 2024 or 7075 may be a little more sensitive to the anodizing parameters. Since alloying elements such as copper are higher, and may lead to an uneven oxide layer and non-uniformity.
2. Surface Preparation
Oils, dirt, or oxidation can disrupt the aluminum oxide layer. In most cases, etching can improve the surface visually, particularly for products that need to be dull.
3. Electrolyte Composition
The electrolyte concentration bath is a major determinant of anodized layer quality. In most anodizing processes, sulfuric acid is the preferred electrolyte. However, design manufacturers can use other acids, such as chromic acid, to attain a particular oxide layer. The acid concentration and the temperature of the electrolyte control the anodizing speed as well as the coating thickness formed in aluminum. For instance, as the temperature decreases, the acid concentration also decreases.
4. Voltage and Current Density
Generally, higher voltages produce thicker oxide coatings. For example, sulfuric acid anodizing usually applies voltage from 12V to 20V for the usual thickness, while the hard coat anodizing applies voltage from 18V to 24V to form a much thicker and tougher oxide layer. Furthermore, the current density determines the uniformity of the oxide layer formation on the aluminum substrate. High current density has effects such as creating non-uniform coatings and also poor surface finish.
5. Temperature Control
Temperature also plays a critical role in the aluminum anodizing process. The temperature of the electrolyte bath is usually in the range between 18°C to 22°C (64°F to 72°F) for sulfuric acid anodizing. Lower temperatures take a longer time to anodize and give less thickness of the coating, but higher temperatures may speed up the reaction and give more thickness of the oxide layers.
6. Coating Thickness
The oxide layer coating thickness proves to be one of the most crucial characteristics because it determines both the strength and functionality of the anodized aluminum. The thickness layers formed through standard anodizing range from 0.0002″ to 0.001″. Type III hard coat anodizing can create oxide coatings of up to 0.003”, which is optimum for abrasion and corrosion, thus best for industrial and aerospace industries.
7. Coloring and Dyeing
Design manufacturers dye anodized aluminum when color is needed because the porous oxide layer absorbs dyes. The color obtained depends on the type of dye used as well as the process parameters of anodizing.
For instance, long anodizing periods typically produce black or bronze coloring and create thicker oxide layers. The porous surface absorbs the dye before the sealing process begins. Then, manufacturers coat the aluminum with copper to set the color and prevent it from fading.
Which Aluminum Grades Are Best Suitable for Anodizing?
Several industries use these aluminum grades in anodizing applications.
- 6061 Aluminum: Manufacturers commonly anodize 6061 alloy because it is strong, durable, and forms a uniform oxide layer. It is also flexible, making it suitable for both structural and ornamental purposes.
- 5052 Aluminum: Alloy 5052 has excellent corrosion resistance, especially in marine environments, and offers weldability. It anodizes well and forms a thick, hard oxide layer, which makes it especially suitable for use in applications that require protection.
- 3003 Aluminum: 3003 has lower strength but good corrosion protection and formability.
- 7075 Aluminum: 7075 is an aluminum-based alloy containing 5.6% aluminum zinc. However, it’s not easy to anodize, but it forms a dense, strong oxide layer that offers good slip and corrosion wear protection.
- 2024 Aluminum: Engineers use 2024 for its high strength and lightweight properties, especially in aerospace and high-performance industries.
How Thick Can Anodized Aluminum Be?
 Anodizing Coating Thickness
Anodizing Coating Thickness
Image Description: A close-up illustration showing different anodizing coating thickness layers on an aluminum surface.
Generally, anodized coatings are usually between 0.0002 and 0.003 inches to 0.005 mm and 0.075 mm in thickness, depending on certain factors. Here’s a breakdown:
- Standard Anodizing (Type 2): The thickness of the coating varies from 0.0002 inches to 0.001 inches (0.005 mm to 0.025 mm) and is suitable for most commercial and aesthetic applications.
- Hard Coat Anodizing (Type 3): In heavily loaded areas, the anodizing process produces oxide layers ranging from 0.01 inch to 0.003 inches (0.025 mm to 0.075 mm) in thickness.
Summary
Aluminum anodizing is a cost-effective, efficient, and adaptable method of finishing aluminum products and increasing their durability, corrosion resistance, and aesthetic value. In case you require components for aerospace, automotive, or any industrial application, anodizing is the best possible solution to increase the performance as well as the looks. However, if you are to appreciate the different types of anodizing, you will be able to produce long-lasting and high-end aluminum products.
Get Premium Anodizing Services for All Your Materials at Premium Parts
Looking for a reliable anodizing service provider? Our primary services include anodizing aluminum, titanium, and other metals, with a range of surface finishes tailored to your needs. Whether you require decorative anodizing, protective coatings, or heavy-duty anodizing, our team ensures precise tolerances and high-performance results every time. So, do not hesitate to contact us to find out how we can assist you in improving your material with anodizing services!